Puremidine The New Longevity Ingredient by NNB Nutrition

You probably haven’t heard of spermidine, but you’ve likely eaten it without knowing. Spermidine can be found in many common foods such as broccoli, cauliflower, mushrooms, soybeans, green peppers, peas, hazelnuts and even some cheeses.1 It can also be produced by the body from the amino acids arginine, ornithine and methionine and can be synthesized in the gut via the intestinal microbiota.2
Biochemically speaking, spermidine belongs to the group of organic compounds called polyamines, along with spermine and putrescine. Polyamines are polycations, which means they have more than two amino groups and can readily interact with negatively charged molecules such as DNA, RNA, proteins and lipids.3 Polyamines can play multiple roles in DNA stability, cell growth and proliferation, as well as cell survival and death.3
 Therefore, changes in polyamine levels, specifically spermidine, have been associated with aging and disease. Spermidine is considered the most abundant polyamine in human tissues, and research suggests that increasing spermidine intake may slow the aging process and promote longevity. High dietary intake of spermidine has been associated with reduced cardiovascular and cancer-related mortality, reduced cognitive impairment and regulation of the inflammatory response, as well as other positive benefits such as gut health, muscle and weight maintenance.
Therefore, changes in polyamine levels, specifically spermidine, have been associated with aging and disease. Spermidine is considered the most abundant polyamine in human tissues, and research suggests that increasing spermidine intake may slow the aging process and promote longevity. High dietary intake of spermidine has been associated with reduced cardiovascular and cancer-related mortality, reduced cognitive impairment and regulation of the inflammatory response, as well as other positive benefits such as gut health, muscle and weight maintenance.
This article will review the function, the overall health protecton and longevity benefits of spermidine, and spermidine supplementation.
The Biochemical Function and Homeostatic Mechanism of Polyamines
To understand the beneficial role of polyamines and spermidine, we must first understand the chemistry and its impact on various biological pathways. Polyamine regulation requires a homeostatic balance, via a cyclic combination of synthesis (or production) and catabolism (or deconstruction) of the polyamines.
As mentioned, polyamines are synthesized from the amino acids arginine, ornithine and methionine. Therefore, the first step is the production of ornithine from arginine by the enzyme arginase. Ornithine is then decarboxylated to form ornithine decarboxylase (ODC); this removal of a carboxyl group and release of carbon dioxide produces the polyamine called putrescine.3
At the same time, the amino acid methionine is decarboxylated via a separate pathway to form decarboxylated S-adenosyl-methionine, which is then used as an aminopropyl group donor to putrescine by spermidine synthase to produce spermidine, or by spermidine to transform into spermine via spermine synthase.3
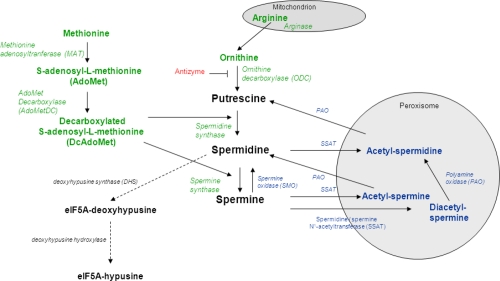 Both spermidine and spermine can be converted back to putrescine via enzymatic processes of spermidine/spermine-acetyltransferase (SSAT). Acetylated spermine and spermidine can then move into the peroxisome, where they are oxidized by polyamine oxidase (PAO). From here, they can loop back into the production of either putrescine or spermidine. This interconversion of polyamines is a cyclic process that controls their turnover and regulates intracellular homeostasis depending on their need and function in the body.
Both spermidine and spermine can be converted back to putrescine via enzymatic processes of spermidine/spermine-acetyltransferase (SSAT). Acetylated spermine and spermidine can then move into the peroxisome, where they are oxidized by polyamine oxidase (PAO). From here, they can loop back into the production of either putrescine or spermidine. This interconversion of polyamines is a cyclic process that controls their turnover and regulates intracellular homeostasis depending on their need and function in the body.
Polyamines’ Metabolic Actions in the Body
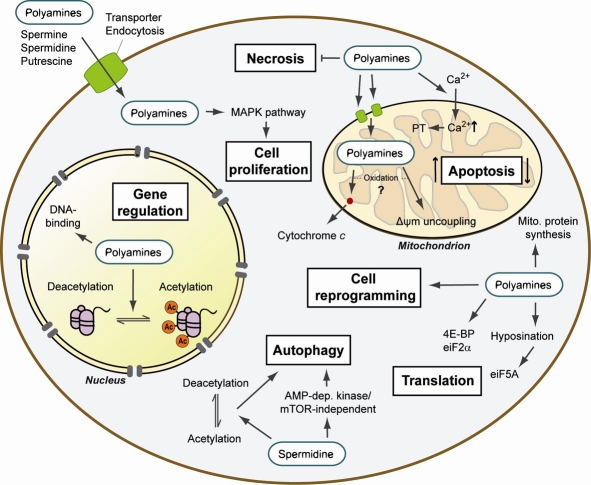
In the cell, polyamines have various functions in the cytoplasm, the nucleus and the powerhouse of the cell, the mitochondria. Polyamines play essential roles in cell growth and proliferation, stabilization of DNA, RNA transcription, protein synthesis, regulation and development of the immune response, regulation of ion channels and the inflammatory response, and as antioxidants, protecting membrane lipids.1 Polyamines also have functions including reprogramming and autophagy regulation. More on that later!
 Polyamine production and content within the cells can be influenced by an individual’s stage of growth and or age. In an animal model, transgenic mice with overexpression of ODC and SSAT were found to have high levels of putrescine and exhibited low levels of spermine and spermidine and were found to have decreased life span.4
Polyamine production and content within the cells can be influenced by an individual’s stage of growth and or age. In an animal model, transgenic mice with overexpression of ODC and SSAT were found to have high levels of putrescine and exhibited low levels of spermine and spermidine and were found to have decreased life span.4
Another study measured polyamine levels in 3-, 10- and 26-week-old mice in various tissues.5 The level of spermidine in the skin was maximal in 10-week-old mice and markedly reduced in 26-week-old mice. In fact, polyamine levels, specifically spermidine, were reduced in 11 of 14 tissues. It isn’t known whether polyamine synthesis or inhibition are affected as one ages, but more studies need to be conducted to explore the effectiveness of supplementation in this area.
In a different study, mice were fed a low-, normal- or high-polyamine diet. Mortality in mice fed a high polyamine diet was lower in the first 88 weeks.6 Additionally, after sacrifice at 88 weeks, those on the high-polyamine diets showed a lower incidence of age-related kidney glomerulosclerosis. This study shows the possibility of oral polyamine supplementation to inhibit the progression of age-associated pathologies.
Cell Autophagy & Spermidine
It’s proposed that one of the main mechanisms of polyamines, specifically spermidine, is autophagy. Autophagy is the process of phagocytosis, which is the ingestion of cytoplasmic proteins or organelles to be recycled into other needed compounds or destroyed. Both autophagy and polyamine levels decrease with age. Deficient autophagy can be linked to many age-related health concerns. Therefore, some research suggests spermidine could be a valuable supplement for longevity. Spermidine has been shown to induce mitophagy, a specific type of autophagy that targets damaged and harmful mitochondria, thus improving abundance of these powerhouse cells.
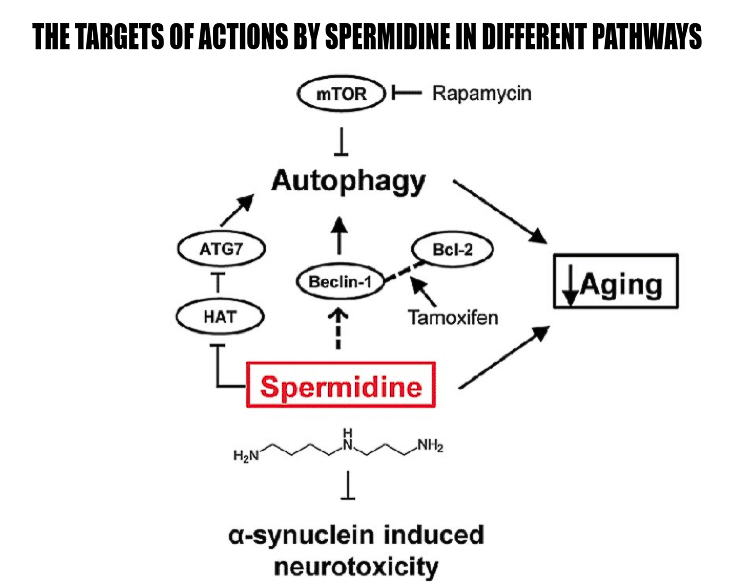 In one study, mice were divided into four groups: 4-month-old mice and 18-month-old mice who drank water either with or without spermidine.7 Spermidine was found to prolong median life span by 10 percent. In addition, spermidine-supplemented mice had higher levels of autophagosome and autolysosome markers in cardiac tissue, whereas age-matched controls without spermidine showed a reduced elevation of this marker, indicating that spermidine increases cardiac autophagy. Spermidine content was significantly increased in mice that received spermidine compared to control subjects, showing that spermidine has high bioavailability.
In one study, mice were divided into four groups: 4-month-old mice and 18-month-old mice who drank water either with or without spermidine.7 Spermidine was found to prolong median life span by 10 percent. In addition, spermidine-supplemented mice had higher levels of autophagosome and autolysosome markers in cardiac tissue, whereas age-matched controls without spermidine showed a reduced elevation of this marker, indicating that spermidine increases cardiac autophagy. Spermidine content was significantly increased in mice that received spermidine compared to control subjects, showing that spermidine has high bioavailability.
Benefits of Spermidine
Supports Cognitive Function
 Spermidine supplementation may have some protective benefits when it comes to cognitive health and function. The neuroprotective effect of spermidine was investigated on SAMP8 (senescence accelerated aging model) mice, using an object recognition test (ORT) to evaluate various aspects of learning and memory, as well as in an open field test (OFT).8
Spermidine supplementation may have some protective benefits when it comes to cognitive health and function. The neuroprotective effect of spermidine was investigated on SAMP8 (senescence accelerated aging model) mice, using an object recognition test (ORT) to evaluate various aspects of learning and memory, as well as in an open field test (OFT).8
After treatment with spermidine, learning and memory were improved in SAMP8 mice. Spermidine significantly improved time of duration of exploring the novel object in the ORT. In addition, spermidine-supplemented mice were more likely to demonstrate better quality and quantity of activity in OFT compared to control subjects.
It’s proposed that this positive impact on cognitive function is via the regulation of cell apoptosis. SAMP8 mice who received spermidine had decreased expression of apoptosis related markers while increasing positive markers at the same time, showing evidence that spermidine may promote healthy brain aging by helping regulate apoptosis.8
In a three-month study in elderly adults with dementia, 85 subjects between 60 and 96 years of age received a daily dose of either 3.3 milligrams of spermidine or 1.9 milligrams of spermidine within a baked bread roll with breakfast six days per week.9 The study used the CERAD-Plus test to evaluate cognitive abilities including verbal fluency, memory, recall and orientation. The results demonstrated a clear correlation between the intake of spermidine and improvement in cognitive performance in subjects with mild and moderate dementia in the group with the higher spermidine dosage.
Has Antioxidant Properties & Improves Inflammatory Response
 Antioxidants can provide protection against oxidation brought on by oxidative stressors. Oxidative stress such as the inflammatory response in the body is a needed response to help kick-start recovery processes; however, excess inflammation can lead to an imbalance of damaging free radicals, including reactive oxygen species (ROS), and the body’s ability to remove them. ROS production is mediated via macrophages and neutrophils in the immune system. Blocking macrophage and neutrophil activation is therefore a potential approach to help promote a healthy inflammatory response and support the body’s natural antioxidant defence.
Antioxidants can provide protection against oxidation brought on by oxidative stressors. Oxidative stress such as the inflammatory response in the body is a needed response to help kick-start recovery processes; however, excess inflammation can lead to an imbalance of damaging free radicals, including reactive oxygen species (ROS), and the body’s ability to remove them. ROS production is mediated via macrophages and neutrophils in the immune system. Blocking macrophage and neutrophil activation is therefore a potential approach to help promote a healthy inflammatory response and support the body’s natural antioxidant defence.
A study investigated the in vitro and in vivo antioxidant potential of spermidine for reducing pro-inflammatory and oxidative effects in lipopolysaccharide (LPS) stimulated macrophages and zebrafish.10 The study found that spermidine attenuated the body’s primary pro-inflammatory signaling pathway and reduced LPS-induced intracellular accumulation of ROS in macrophages. In addition, spermidine prevented LPS-induced production of nitric oxide (NO), an inflammatory mediator. Spermidine was also found to downregulate LPS-induced NO and ROS production in zebrafish. This study suggests that spermidine may offer antioxidant protective effects that promote a healthy inflammatory response.
Spermidine may also mediate the inflammatory response via other mechanisms. In spermidine-treated 18-month-old mice, levels of interleukin-6 (IL-6), a pro-inflammatory cytokine, were reduced, and mitochondrial function was improved within the aorta. This was accompanied by reduced atherogenesis.11 This study shows that spermidine may help to reduce inflammation and age-related atherosclerosis.
Lowers Mortality Rate
 A long-term prospective population study evaluated the relationship between dietary spermidine intake and mortality, in 829 participants between the ages of 45 and 85.12 In this 20-year study, over 2,500 diet food-frequency assessments were collected, and the results showed that all-cause mortality decreased with higher spermidine intake, even when controlling for age, sex, caloric intake and lifestyle factors.12
A long-term prospective population study evaluated the relationship between dietary spermidine intake and mortality, in 829 participants between the ages of 45 and 85.12 In this 20-year study, over 2,500 diet food-frequency assessments were collected, and the results showed that all-cause mortality decreased with higher spermidine intake, even when controlling for age, sex, caloric intake and lifestyle factors.12
The reduction in mortality risk related to a diet rich in spermidine was comparable to that associated with a 5.7-year younger age, when compared with the lower third of spermidine intake. Researchers identified foods containing high amounts of spermidine such as broccoli, mushrooms, cheeses, mushrooms and green peppers.
Maintains Skeletal Muscle
 Evidence suggests that polyamines in the muscle tissue may have a protective effect. It’s known that skeletal muscle reduction occurs with aging. Spermidine’s promotion of autophagy may support maintenance of skeletal muscle mass. One study examined the effects of spermidine and exercise on skeletal muscle maintenance in aging rats.
Evidence suggests that polyamines in the muscle tissue may have a protective effect. It’s known that skeletal muscle reduction occurs with aging. Spermidine’s promotion of autophagy may support maintenance of skeletal muscle mass. One study examined the effects of spermidine and exercise on skeletal muscle maintenance in aging rats.
The study used rats whose aging was artificially accelerated via injection of D-galactose (D-gal). These rats received 5 milligrams of spermidine per kilogram per day, swam 60 minutes a day for five days a week, or did a combination of both for 42 days.13 Results showed that spermidine with exercise could reduce D-gal-induced aging-related losses in skeletal muscle by inducing autophagy and helping regulate apoptosis with characteristics of more autophagosomes, activated mitophagy, enhanced mitochondrial quality, alleviated cell shrinkage and less swollen mitochondria.
Promotes Weight Maintenance & Thermogenesis
 Some research indicates that spermidine promotes healthy weight management. In fact, both spermidine and spermine are essential factors at early stages of adipocyte differentiation as they modulate the expression levels of transcriptional factors implicated in the adipogenesis regulation. What’s more, low dietary spermidine intake has been correlated to obesity and poor metabolic health, suggesting that spermidine intake might be a factor that contributes to the prevalence of obesity.14
Some research indicates that spermidine promotes healthy weight management. In fact, both spermidine and spermine are essential factors at early stages of adipocyte differentiation as they modulate the expression levels of transcriptional factors implicated in the adipogenesis regulation. What’s more, low dietary spermidine intake has been correlated to obesity and poor metabolic health, suggesting that spermidine intake might be a factor that contributes to the prevalence of obesity.14
One study evaluated the impact of spermidine on high-fat diet (HFD) induced obese mice.14 Mice were fed a HFD for 16 weeks, then divided into two groups: One group continued to only receive an HFD, while the other was given both a HFD and spermidine in drinking water. This study found that obese mice that received spermidine had lower fat mass and plasma lipid levels, but no changes in body weight were examined. In addition, spermidine reduced hepatic steatosis, also known as fatty liver, via regulation of lipid metabolism and enhancing antioxidant capacity.
Spermidine-treated mice also experienced reduced adipose tissue inflammation, via decreased inflammatory cytokine response. Lastly, the study found that spermidine also promoted brown adipose tissue (BAT) thermogenesis, thus increasing energy expenditure. This study shows the potential of spermidine to regulate fat metabolism, inflammation and potentially thermogenesis for the treatment of obesity.
Supports Healthy Gut Function
 Healthy gut microbial balance and function are important for gut health, metabolic health and the production of short-chain fatty acids, such as butyrate, that can affect metabolic health, appetite-regulating hormones and more. The effects of spermidine supplementation on gut microbiota were evaluated in eight-week-old mice.15 The mice were given a HFD for 16 weeks then divided into two groups: only HFD or HFD with 20 milligrams per kilogram per day of spermidine in drinking water.
Healthy gut microbial balance and function are important for gut health, metabolic health and the production of short-chain fatty acids, such as butyrate, that can affect metabolic health, appetite-regulating hormones and more. The effects of spermidine supplementation on gut microbiota were evaluated in eight-week-old mice.15 The mice were given a HFD for 16 weeks then divided into two groups: only HFD or HFD with 20 milligrams per kilogram per day of spermidine in drinking water.
Spermidine prevented a microbial shift and helped with gut barrier protection via increased expression of autophagy and decreased expression of apoptosis. It also reduced inflammation in the colon and increased mucus secretion. Furthermore, an increase of 4.4 percent of Firmicutes and a decrease of Bacteroidetes by 4.2 percent was observed in mice fed the HFD, which were rescued by spermidine.15
Supplementation & Dosage of Puremidine
Although limited human research has been done on spermidine supplementation and its potential benefits, some evidence suggests its effectiveness via consumption of polyamine-rich  foods, in addition to the many positive outcomes in animal models. NNB Nutrition, an industry-leading ingredient provider, is launching a pure, high-quality spermidine called Puremidine. With one to three servings per day of 0.3 to 0.4 milligrams ingested at mealtime, this ingredient will have the potential to maintain muscle, improve weight, improve gut health, reduce inflammation and act as a potent antioxidant via spermidine’s autophagic mechanism. Watch out for Puremidine to be formulated in sports nutrition, weight maintenance and longevity products.
foods, in addition to the many positive outcomes in animal models. NNB Nutrition, an industry-leading ingredient provider, is launching a pure, high-quality spermidine called Puremidine. With one to three servings per day of 0.3 to 0.4 milligrams ingested at mealtime, this ingredient will have the potential to maintain muscle, improve weight, improve gut health, reduce inflammation and act as a potent antioxidant via spermidine’s autophagic mechanism. Watch out for Puremidine to be formulated in sports nutrition, weight maintenance and longevity products.

 Want to know more about NNB Nutrition and Puremidine? Visit www.nnbnutrition.com.
Want to know more about NNB Nutrition and Puremidine? Visit www.nnbnutrition.com.
References
- Muñoz-Esparza NC, Latorre-Moratalla ML, Comas-Basté O, et al. Polyamines in food. Front Nutr. 2019 Jul 11;6:108. doi: 10.3389/fnut.2019.00108.
- Noack J, Dongowski G, Hartmann L, Blaut M. The human gut bacteria Bacteroides thetaiotaomcron and Fusobacterium varium produce putrescine and spermidine in cecum of pectin-fed gnotobiotic rats. J Nutr. 2000 May;130(5):1225-31. doi: 10.1093/jn/130.5.1225.
- Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY). 2011 Aug;3(8):716-32. doi: 10.18632/aging.100361.
- Suppola S, Heikkinen S, Parkkinen JJ, et al. Concurrent overexpression of ornithine decarboxylase and spermidine/spermine N(1)-acetyltransferase further accelerates the catabolism of hepatic polyamines in transgenic mice. Biochem J. 2001 Sep 1;358(Pt 2):343-8. doi: 10.1042/0264-6021:3580343.
- Nishimura K, Shiina R, Kashiwagi K, Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem. 2006 Jan;139(1):81-90. doi: 10.1093/jb/mvj003.
- Polyamine-rich food decreases age-associated pathology and mortality in aged mice.
- Soda K, Dobashi Y, Kano Y, Tsujinaka S, Konishi F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp Gerontol. 2009 Nov;44(11):727-32. doi: 10.1016/j.exger.2009.08.013.
- Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016 Dec;22(12):1428-1438. doi: 10.1038/nm.4222.
- Xu TT, Li H, Dai Z, et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging (Albany NY). 2020 Apr 8;12(7):6401-6414. doi: 10.18632/aging.103035.
- Pekar T, Bruckner K, Pauschenwein-Frantsich S, et al. The positive effect of spermidine in older adults suffering from dementia. Wien Klin Wochenschr. 2021 May;133(9-10):484-491. doi: 10.1007/s00508-020-01758-y.
- Jeong JW, Cha HJ, Han MH, et al. Spermidine protects against oxidative stress in inflammation models using macrophages and zebrafish. Biomol Ther (Seoul). 2018 Mar 1;26(2):146-156. doi: 10.4062/biomolther.2016.272.
- Tyrrell DJ, Blin MG, Song J, et al. Age-associated mitochondrial dysfunction accelerates atherogenesis. Circ Res. 2020 Jan 31;126(3):298-314. doi: 10.1161/CIRCRESAHA.119.315644.
- Kiechl S, Pechlaner R, Willeit P, et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr. 2018 Aug 1;108(2):371-380. doi: 10.1093/ajcn/nqy102.
- Fan J, Yang X, Li J, et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget. 2017 Mar 14;8(11):17475-17490. doi: 10.18632/oncotarget.15728.
- Ma L, Ni Y, Hu L, et al. Spermidine ameliorates high-fat diet-induced hepatic steatosis and adipose tissue inflammation in pre-existing obese mice. Life Sci. 2021 Jan 15;265:118739. doi: 10.1016/j.lfs.2020.118739.
- Ma L, Ni Y, Wang Z, et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes. 2020 Nov 9;12(1):1-19. doi: 10.1080/19490976.2020.1832857.

